In 2011, about a month after joining the MIT faculty, Feng Zhang attended a talk by Harvard Medical School Professor Michael Gilmore, who studies the pathogenic bacterium Enteroccocus. The scientist mentioned that these bacteria protect themselves from viruses with DNA-cutting enzymes known as nucleases, which are part of a defense system known as CRISPR.
“I had no idea what CRISPR was but I was interested in nucleases,” Zhang says. “I went to look up CRISPR, and that’s when I realized you might be able to engineer it for use for genome editing.”
Zhang devoted himself to adapting the system to edit genes in mammalian cells and recruited new members to his nascent lab at the Broad Institute of MIT and Harvard to work with him on this project. In January 2013, they reported their success in the journal Science.
Since then, scientists in fields from medicine to plant biology have begun using CRISPR to study gene function and investigate the possibility of correcting faulty genes that cause disease. Zhang now heads a lab of 19 scientists who continue to develop the system and pursue applications of genome editing, especially in neuroscience.
“The goal is to try to make our lives better by developing new technologies and using them to understand biological systems so that we can improve our treatment of disease and our quality of life,” says Zhang, W. M. Keck Career Development Associate Professor in Biomedical Engineering and a member of MIT’s McGovern Institute for Brain Research. Zhang recently earned tenure in MIT’s Departments of Biological Engineering and Brain and Cognitive Sciences.
Understanding the brain
Growing up in Des Moines, Iowa, where his parents moved from China when he was 11, Zhang had plenty of opportunities to feed his interest in science. He participated in Science Bowl competitions and took special Saturday science classes, where he got his first introduction to molecular biology. Experiments such as extracting DNA from strawberries and transforming bacteria with genes for drug resistance whetted his appetite for genetic engineering, which was further stimulated by a showing of “Jurassic Park.”
“That really caught my attention,” he recalls. “It didn’t seem that far-fetched. I guess that’s what makes it good science fiction. It kind of tantalizes your imagination.”
As a sophomore in high school, Zhang began working with Dr. John Levy in a gene therapy lab at the Iowa Methodist Medical Center in Des Moines, where he studied green fluorescent protein (GFP). Scientists had recently figured out how to adapt this naturally occurring protein to tag and image proteins inside living cells. Zhang used it to track viral proteins within infected cells to determine how the proteins assemble to form new viruses. He also worked on a project to adapt GFP for a different purpose — protecting DNA from damage induced by ultraviolet light.
At Harvard University, where he earned his undergraduate degree, Zhang majored in chemistry and physics and did research under the mentorship of Xiaowei Zhuang, a professor of chemistry and chemical biology. “I was always interested in biology but I felt that it’s important to get a solid training in chemistry and physics,” he says.
While Zhang was at Harvard, a close friend was severely affected by a psychiatric disorder. That experience made Zhang think about whether such disorders could be approached just like cancer or heart disease, if only scientists knew more about their underlying causes.
“The difference is we’re at a much earlier stage of understanding psychiatric diseases. That got me really interested in trying to understand more about how the brain works,” he says.
At Stanford University, where Zhang earned his PhD in chemistry, he worked with Karl Deisseroth, who was just starting his lab with a focus on developing new technology for studying the brain. Zhang was the second student to join the lab, and he began working on a protein called channelrhodopsin, which he and Deisseroth believed held potential for engineering mammalian cells to respond to light.
The resulting technique, known as optogenetics, has transformed biological research. Collaborating with Edward Boyden, a member of the Deisseroth lab who is now a professor at MIT, Zhang adapted channelrhodopsin so that it could be inserted into neurons and make them light-sensitive. Using this approach, neuroscientists can now selectively activate and de-activate specific neurons in the brain, allowing them to map brain circuits and investigate how disruption of those circuits causes disease.
Better gene editing
After leaving Stanford, Zhang spent a year as a junior fellow at the Harvard Society of Fellows, studying brain development with Professor Paola Arlotta and collaborating with Professor George Church. That’s when he began to focus on gene editing — a type of genetic engineering that allows researchers to selectively delete a gene or replace it with a new one.
He began with zinc finger nucleases — enzymes that can be designed to target and cut specific DNA sequences. However, these proteins turned out to be challenging to work with, in part because it is so time-consuming to design a new protein for each possible DNA target.
That led Zhang to experiment with a different type of nucleases known as transcription activator-like effector nucleases (TALENs), but these also proved laborious to work with. “Learning how to use them is a project on its own,” Zhang says.
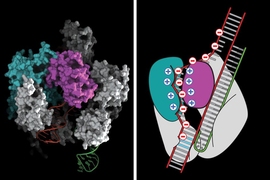
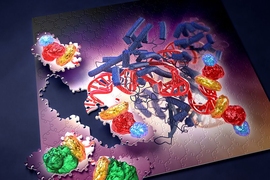
When he heard about CRISPR in early 2011, Zhang sensed that harnessing the natural bacterial process held the potential to solve many of the challenges associated with those earlier gene-editing techniques. CRISPR includes a nuclease called Cas9, which can be guided to the correct genetic target by RNA molecules known as guide strands. For each target, scientists need only design and synthesize a new RNA guide, which is much simpler than creating new TALEN and zinc finger proteins.
Since his first CRISPR paper in 2013, Zhang’s lab has devised many enhancements to the original system, such as making the targeting more precise and preventing unintended cuts in the wrong locations. They also recently reported another type of CRISPR system based on a different nuclease called Cpf1, which is simpler and has unique features that further expand the genome editing toolbox.
Zhang’s lab has become a hub for CRISPR research worldwide. It has shared CRISPR-Cas9 components in response to nearly 30,000 requests from academic laboratories around the world and has trained thousands of researchers in the use of CRISPR-Cas9 genome-editing technology through in-person events and online opportunities.
His team is now working on creating animal models of autism, Alzheimer’s, and other neurological disorders, and in the long term, they hope to develop CRISPR for use in humans to potentially cure diseases caused by defective genes.
“There are many genetic diseases that we don’t have any way of treating and this could be one way, but we still have to do a lot of work,” Zhang says.