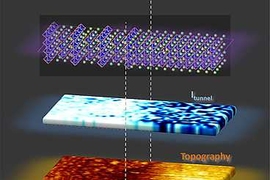
Pyrite — perhaps better known as “fool’s gold” for its yellowish metallic appearance — is a common, naturally occurring mineral. It holds promise as a high-tech material, with potential uses in solar cells, spintronic devices and catalysts, but is also a byproduct of corrosion of steel in deep-sea oil and gas wells. Both its potential usefulness in devices and its role in corrosion are largely influenced by the fundamental electronic properties of its surface — which have remained relatively unexplored.
But a team of MIT researchers has now found a way to probe these elusive surface properties for the first time. Their findings are reported in the journal Surface Science, in a paper by professors Bilge Yildiz and Krystyn Van Vliet and graduate students F. William Herbert and Aravind Krishnamoorthy.
“The surface of this material is very different from the bulk, something that is common for many materials,” Yildiz explains. “The bulk has been widely characterized, but when it comes to the surface, there is only a small amount of data, and it’s not consistent.”
Yet in reaping the benefits of pyrite, “it’s important to know about the surface,” she says.
Measuring the surface electronic characteristics of materials is technically much more challenging than measuring their bulk properties — difficulties that arise from “a combination of the available tools as well as the characteristics of the surfaces of materials,” says Yildiz, an associate professor of nuclear science and engineering.
The new work was made possible by combining scanning tunneling spectroscopy (STS), a tool developed in the 1980s, with modern computational methods to interpret its output, Herbert explains. “You need a tool that is measuring just to a shallow depth of one or two atomic layers” on a sample, he says, but also the computational ability to establish “how the surface behaves differently from the bulk, and how to model the experimental data.”
The new measurements reveal, among other things, that on pyrite’s surface, a property called an energy bandgap — essential for making solar cells or semiconductor devices — has a value less than half that of the bulk material. Previous studies had produced conflicting results for the bandgap at the surface. In order to uncover the true nature of this surface, Herbert performed tunneling spectroscopy measurements on pristine pyrite surfaces, and analyzed his results with theoretical modeling of the tunneling spectra. The model was adapted from semiconductor physics, and informed by Krishnamoorthy’s electronic structure calculations.
The reason for the discrepancy between surface and bulk properties, Yildiz explains: “The surface has dangling bonds, providing electronic states into the bulk bandgap.”

Pyrite, or iron sulfide (FeS2), forms in the oxygen-free environment of the deep ocean. It tends to form a corrosive coating on steel structures such as the casings of oil and gas wells, so oil companies are interested in better understanding the material’s properties so as to develop means of slowing the corrosion. (BP supported the research, through the BP-MIT Center for Materials and Corrosion Research.)
As oil wells move into deeper and deeper waters, the prevalence of pyrite will increase, Yildiz says. “Nearer the ocean surface, where oxygen is plentiful, these metals can form what we commonly call rust or iron oxide, but at depth, with little oxygen, you get iron sulfide instead,” explains Van Vliet, an associate professor of materials science and engineering.
“A smaller bandgap leads to more corrosion,” Yildiz adds, so “if people based countermeasures and models on the properties tested for the bulk material, they would underestimate the corrosion rate.”
Pyrite has significant potential in its own right, the researchers say: Its possible use as a material for solar cells was investigated as far back as the 1980s. The new findings don’t solve pyrite’s low-bandgap problem on its surface, but they do “explain why it works so poorly, despite the expected high performance based on the bandgap of the bulk material,” Van Vliet says. “There could be ways to treat the surface to eliminate the problem,” Yildiz suggests.
If so, pyrite could find significant use in solar cells, Van Vliet says: “The material itself is abundant and inexpensive.” It has also been suggested as a possible material for spintronic devices, in which the spin of electrons carries information.
The techniques the team used, Yildiz and Van Vliet say, could also be used to understand the differences between surface and bulk characteristics in other materials. “This is a way of characterizing the bandgap of materials generally that was not taken full advantage of before,” they say.
Randall Feenstra, a professor of physics at Carnegie Mellon University who was not part of this research team, says that while the basic techniques used by the MIT researchers have been applied to other materials, “The uniqueness of the work here is that the application of the methodology, combining experiment and theory, went well beyond what was done in other materials.” He says this “excellent work … will certainly find application to surfaces of other materials.”