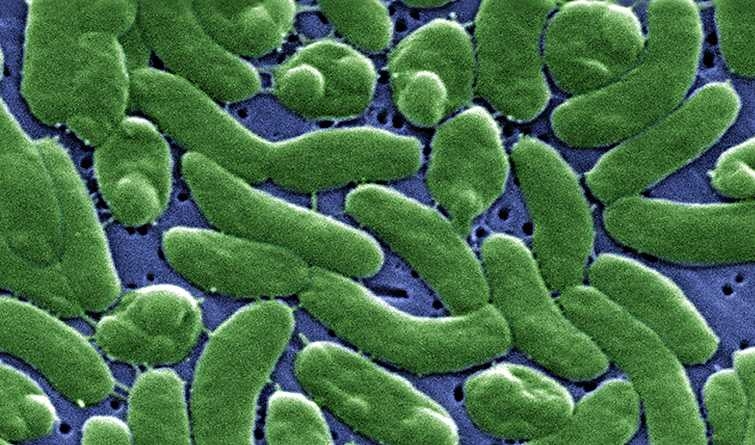
Bacteria are the most populous organisms on the planet: They thrive in almost every known environment, adapting to different habitats by means of genetic variations that provide the capabilities essential for survival. These genetic innovations arise from what scientists believe is a random mutation and exchange of genes and other bits of DNA among bacteria that sometimes confers an advantage, and which then becomes an intrinsic part of the genome.
But how an advantageous mutation spreads from a single bacterium to all the other bacteria in a population is an open scientific question. Does the gene containing an advantageous mutation pass from bacterium to bacterium, sweeping through an entire population on its own? Or does a single individual obtain the gene, then replicate its entire genome many times to form a new and better-adapted population of identical clones? Conflicting evidence supports both scenarios.
In a paper appearing in the April 6 issue of Science, researchers in MIT’s Department of Civil and Environmental Engineering (CEE) provide evidence that advantageous mutations can sweep through populations on their own. The study reconciles the previously conflicting evidence by showing that after these gene sweeps, recombination becomes less frequent between bacterial strains from different populations, yielding a pattern of genetic diversity resembling that of a clonal population.
This indicates that the process of evolution in bacteria is very similar to that of sexual eukaryotes — which do not pass their entire genome intact to their progeny — and suggests a unified method of evolution for Earth’s two major life forms: prokaryotes and eukaryotes.
The findings also get to the heart of another scientific question: how to delineate species of bacteria — or determine if the term “species” even applies to bacteria, which are typically identified as ecological populations and not species. If all bacteria in a population are clones from a common ancestor, the idea of species doesn’t apply. But if, as this new study shows, genes randomly shared among individuals can bring about a new, ecologically specialized population, use of the term may be warranted.
“We found that the differentiation between populations was restricted to a few small patches in the genome,” says Eric Alm, the Karl Van Tassel (1925) Career Development Associate Professor of Civil and Environmental Engineering and Biological Engineering and an associate member of the Broad Institute.
Professor Martin Polz of CEE, co-principal investigator on the project, adds, “Similar patterns have been observed in animals, but we didn’t expect to see it in bacteria.”
The process of ecological differentiation in bacteria, the researchers found, is similar to that in malaria-transmitting mosquitoes: Some populations develop resistance to antimalarial agents by means of a single gene sweep, while other populations sharing the same habitat do not. The stickleback fish has also been shown to follow this pattern of “sympatric speciation” in shared habitats.
“Even though the sources of genetic diversity are quite different between bacteria and sexual eukaryotes, the process by which adaptive diversity spreads and triggers ecological differentiation seems very similar,” says first author Jesse Shapiro PhD ’10, a postdoc at Harvard University who did his graduate work in Alm’s lab at MIT.
The researchers performed the work using 20 complete genomes of the bacterium Vibrio cyclitrophicus that had recently diverged into two ecological populations adapted to microhabitats containing different types of zooplankton, phytoplankton, and suspended organic particles in seawater. In a previous study based on just a few marker genes, they had predicted that these closely related Vibrio populations were in the process of developing into two distinct habitat-associated populations.
The new study shows that the two populations were frequently mixed by genetic recombination, remaining genetically distinct at just a handful of ecologically adaptive genes, with an increasing trend toward gene-sharing within — rather than between — habitats.
“This is the most sophisticated paper on bacterial speciation to appear yet, all the more so because it uses the dubious word ‘species’ only once, and that with caution,” says W. Ford Doolittle, a professor emeritus of biochemistry at Dalhousie University in Canada. “The genetic basis of ecological differentiation in bacteria — how genotype maps to ecotype and what processes determine this mapping — is in my mind the biggest issue in modern microbial ecology.”
Other co-authors on the paper are MIT graduate student Jonathan Friedman, postdocs Otto Cordero and Sarah Preheim, graduate student Sonia Timberlake, and Gitta Szabo of the University of Vienna in Austria. Funding was provided by the National Science Foundation, the Gordon and Betty Moore Foundation, and the Broad Institute.
But how an advantageous mutation spreads from a single bacterium to all the other bacteria in a population is an open scientific question. Does the gene containing an advantageous mutation pass from bacterium to bacterium, sweeping through an entire population on its own? Or does a single individual obtain the gene, then replicate its entire genome many times to form a new and better-adapted population of identical clones? Conflicting evidence supports both scenarios.
In a paper appearing in the April 6 issue of Science, researchers in MIT’s Department of Civil and Environmental Engineering (CEE) provide evidence that advantageous mutations can sweep through populations on their own. The study reconciles the previously conflicting evidence by showing that after these gene sweeps, recombination becomes less frequent between bacterial strains from different populations, yielding a pattern of genetic diversity resembling that of a clonal population.
This indicates that the process of evolution in bacteria is very similar to that of sexual eukaryotes — which do not pass their entire genome intact to their progeny — and suggests a unified method of evolution for Earth’s two major life forms: prokaryotes and eukaryotes.
The findings also get to the heart of another scientific question: how to delineate species of bacteria — or determine if the term “species” even applies to bacteria, which are typically identified as ecological populations and not species. If all bacteria in a population are clones from a common ancestor, the idea of species doesn’t apply. But if, as this new study shows, genes randomly shared among individuals can bring about a new, ecologically specialized population, use of the term may be warranted.
“We found that the differentiation between populations was restricted to a few small patches in the genome,” says Eric Alm, the Karl Van Tassel (1925) Career Development Associate Professor of Civil and Environmental Engineering and Biological Engineering and an associate member of the Broad Institute.
Professor Martin Polz of CEE, co-principal investigator on the project, adds, “Similar patterns have been observed in animals, but we didn’t expect to see it in bacteria.”
The process of ecological differentiation in bacteria, the researchers found, is similar to that in malaria-transmitting mosquitoes: Some populations develop resistance to antimalarial agents by means of a single gene sweep, while other populations sharing the same habitat do not. The stickleback fish has also been shown to follow this pattern of “sympatric speciation” in shared habitats.
“Even though the sources of genetic diversity are quite different between bacteria and sexual eukaryotes, the process by which adaptive diversity spreads and triggers ecological differentiation seems very similar,” says first author Jesse Shapiro PhD ’10, a postdoc at Harvard University who did his graduate work in Alm’s lab at MIT.
The researchers performed the work using 20 complete genomes of the bacterium Vibrio cyclitrophicus that had recently diverged into two ecological populations adapted to microhabitats containing different types of zooplankton, phytoplankton, and suspended organic particles in seawater. In a previous study based on just a few marker genes, they had predicted that these closely related Vibrio populations were in the process of developing into two distinct habitat-associated populations.
The new study shows that the two populations were frequently mixed by genetic recombination, remaining genetically distinct at just a handful of ecologically adaptive genes, with an increasing trend toward gene-sharing within — rather than between — habitats.
“This is the most sophisticated paper on bacterial speciation to appear yet, all the more so because it uses the dubious word ‘species’ only once, and that with caution,” says W. Ford Doolittle, a professor emeritus of biochemistry at Dalhousie University in Canada. “The genetic basis of ecological differentiation in bacteria — how genotype maps to ecotype and what processes determine this mapping — is in my mind the biggest issue in modern microbial ecology.”
Other co-authors on the paper are MIT graduate student Jonathan Friedman, postdocs Otto Cordero and Sarah Preheim, graduate student Sonia Timberlake, and Gitta Szabo of the University of Vienna in Austria. Funding was provided by the National Science Foundation, the Gordon and Betty Moore Foundation, and the Broad Institute.