But Shuguang Zhang, associate director of MIT’s Center for Biomedical Engineering, and postdocs Iftach Yacoby and Sergii Pochekailov, together with colleagues at Tel Aviv University in Israel and the National Renewable Energy Laboratory in Colorado, have found a way to use bioengineered proteins to flip this preference, allowing more hydrogen to be produced.
“The algae are really not interested in producing hydrogen, they want to produce sugar,” Yacoby says — the sugar is what they need for their own survival, and the hydrogen is just a byproduct. But a multitasking enzyme, introduced into the liquid where the algae are at work, both suppresses the sugar production and redirects the organisms’ energies into hydrogen production. The work is described in a paper being published online this week in the Proceedings of the National Academy of Sciences, and was supported in part by a European Molecular Biology Organization postdoctoral fellowship, the Yang Trust Fund and the U.S. Department of Energy’s National Renewable Energy Laboratory.
Adding the bioengineered enzyme increases the rate of algal hydrogen production by about 400 percent, Yacoby says. The sugar production is suppressed but not eliminated, he explains, because “if it went to zero, it would kill the organism.”
The research demonstrates for the first time how the two processes carried out by algae compete with each other; it also shows how that competition could be modified to favor hydrogen production in a laboratory environment. Zhang and Yacoby plan to continue developing the system to increase its efficiency of hydrogen production.
“It’s one step closer to an industrial process,” Zhang says. “First, you have to understand the science” — which has been achieved through this experimental work. Now, developing it further — through refinements to produce a viable commercial system for hydrogen-fuel manufacturing — is “a matter of time and money,” Zhang says. “We’ve had minimal money to come this far,” he says. “”With more money, we could do it faster”

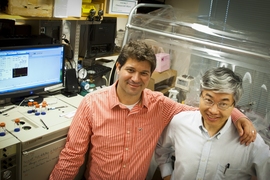
Postdoc Iftach Yacoby, left, and Shuguang Zhang, associate director of MIT’s Center for Biomedical Engineering, stand in front of their experimental setup.
Photo: Patrick Gillooly
Bengt Nordén, chair of the physical chemistry department at the Chalmers University of Technology in Gothenburg, Sweden, says, “The results are convincing, although it is difficult to judge, at this stage, how useful this system could be for practical energy purposes.”
Nordén adds, “Hydrogen will no doubt be the future’s fuel,” either in pure form or combined to make methane. This particular method of making hydrogen, he says, “is a both simple and environmentally friendly bio-inspired system.”
In the long run, “the only viable way to produce renewable energy is to use the sun, [either] to make electricity or in a biochemical reaction to produce hydrogen,” Yacoby says. “I believe there is no one solution,” he adds, but rather many different approaches depending on the location and the end uses.
This particular approach, he says, is simple enough that it has promise “not just in industrialized countries, but in developing countries as well” as a source of inexpensive fuel. The algae needed for the process exist everywhere on Earth, and there are no toxic materials involved in any part of the process, he says.
“The beauty is in its simplicity,” he says.