MIT cancer researchers have discovered a process that may explain how some tumor cells form, a discovery that could one day lead to new therapies that prevent defective cells from growing and spreading.
The work was reported June 8 in the advance online issue of The EMBO Journal, a publication of the European Molecular Biology Organization (EMBO).
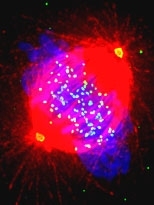
Tumor cells that grow aggressively often have an irregular number of chromosomes, the structures in cells that carry genetic information. The normal number of chromosomes in a human cell is 46, or 23 pairs. Aggressive tumor cells often have fewer or more than 23 pairs of chromosomes, a condition called aneuploidy.
To date it has not been clear how tumor cells become aneuploid.
"Checkpoint proteins" within cells work to prevent cells from dividing with an abnormal number of chromosomes, but scientists have been puzzled by evidence that aneuploidy can result even when these proteins appear to be normal.
What MIT researchers have discovered is a reason these checkpoint proteins may be unable to sense the defective cells, which tend to have very subtle errors in them. (These subtle errors are believed to be the cause of aneuploidy and the rapid growth of tumors.)
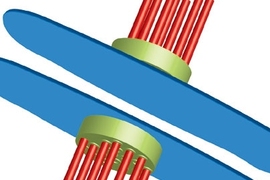
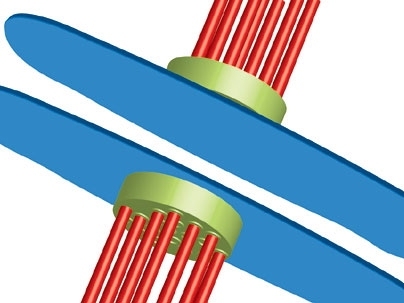
Before cells divide, individual chromosomes in each pair of chromosomes must attach to a set of tiny structures called microtubules. If they attach correctly, the checkpoint proteins give them the go-ahead to divide. If they don't, the checkpoint proteins are supposed to stop them from dividing.
"The checkpoint proteins are like referees in a tug-of-war contest," said Viji Draviam, a research scientist in MIT's Department of Biology and lead author of the paper. "They make sure that all chromosomes are lined up in the right places before the cell is allowed to divide."
Scientists have known about the function of checkpoint proteins for at least 20 years, and they have suspected that mutations in checkpoint proteins cause the irregular number of chromosomes in the aneuploid cells. But they have been perplexed by the infrequent occurrence of mutations in aneuploid tumors.
"It's puzzling that the suspected culprits -- the aneuploidy-inducing checkpoint mutations -- are rarely found at the scene of the crime, in the aneuploid tumors," Draviam said.
That lingering question prompted Draviam and her colleagues to study how two other key molecules -- a known tumor suppressor protein called APC and its partner protein EB1 -- work together to assure that cells divide normally.
They discovered that if they removed either protein from a cell or if they interrupted the way the proteins work together, the cell would become aneuploid. In other words, the checkpoint proteins need to sense that the APC and EB1 proteins both are present for normal cell division to take place.
"This is important because it is the first demonstration that interrupting the normal function of these proteins will cause the cell to become aneuploid," Draviam said. "Our research sheds light on what could go wrong to cause an irregular number of chromosomes in cells even when the checkpoint proteins appear to be functioning properly."
Draviam's co-authors are graduate students Irina Shapiro and Bree Aldridge and MIT Professor of Biology and Biological Engineering Peter Sorger.
The research was funded by the National Institutes of Health.