A gene expressed only in brain areas responsible for high-level thinking and feeling may be key to the brain's ability to respond rapidly to new input, scientists at MIT's Picower Center for Learning and Memory and colleagues report in the Nov. 18 issue of Neuron. The finding may one day allow researchers to manipulate the level or speed at which people learn new information.
Elly Nedivi, the Fred and Carole Middleton Assistant Professor at MIT, Jeffrey Cottrell (MIT Ph.D. 2004), and colleagues from Yale University have identified for the first time a gene encoding a protein that may function as a modulating switch to fine-tune certain neurons' plasticity, or ability to change. In addition to shedding light on plasticity, understanding this protein may one day allow researchers to manipulate the level or speed at which people learn new information.
Neurons pass along information across a small gap called a synapse. The synapse consists of the neuron's presynaptic and postsynaptic endings and a space between them. The postsynaptic ending is dotted with neurotransmitter receptors that act like tiny receivers for chemical signals. Through a mechanism that is not well understood, these receptors appear and disappear. Maintaining these surface receptors is a critical aspect of neuronal function. More receptors allow increased cell activity, but too much activity can be toxic.
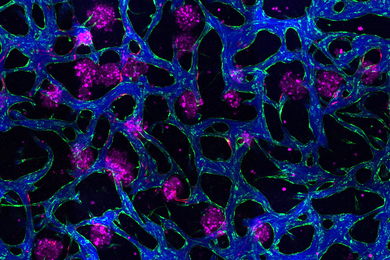
Nedivi's results suggest that candidate plasticity gene 2 (cpg2) and the protein it encodes--CPG2--are key in balancing receptor turnover.
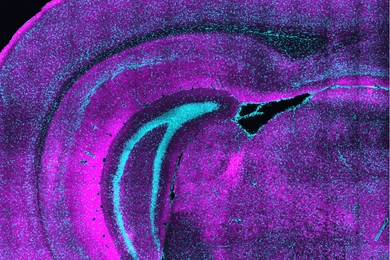
The protein encoded by the candidate plasticity gene 2 exists only near synapses in the parts of the brain responsible for receiving and interpreting sensory information, analyzing information, reasoning, experiencing emotions and initiating movement. This gene "might be the link between levels of brain activity and the ability of the brain to respond to an increased level of activity," said Nedivi, who explores activity-regulating genes that are the driving force behind plasticity--or change--in the brain.
Endocytosis is a process through which a substance can get into a cell without passing through the cell membrane. The cell engulfs a protein and creates a little pouch, or vesicle, to hold it internally. The protein CPG2 regulates endocytosis, controlling the number of receptors at the postsynaptic site. The researchers observed the effects of knocking out the cpg2 gene. "Getting rid of CPG2 clearly perturbs the process," Nedivi said. In the absence of the protein, the number of vesicles increased, decreasing the number of receptors on the surface.
The more active the cell, the more CPG2 is used to clear the vesicles and internalize them at a faster rate, leaving fewer receptors on the surface. This could be a protective mechanism for the synapse, which would be overwhelmed with a too-high level of activity.
In addition to Nedivi and Cottrell, co-authors are Erzsebet Borok and Tamas L. Horvath of Yale University Medical School.
This work is supported by the National Eye Institute, the National Center for Research Resources and the National Institute of Diabetes and Digestive and Kidney Diseases.